Results and discussion (continued)
CN(X 2Σ+) production process
Observing CN products in the present study, we conclude that the insertion process forming the
CF3-O-(CN)intermediate unambiguously occurs in the reaction of O(1D) + CF3CN. Why this intermediate is not decomposed into the CF3 + NCO? We suppose that the electronic state of the intermediate does not correlate adiabatically with the potential energy surface (PES) leading to CF3 + NCO.
Electronic quenching process
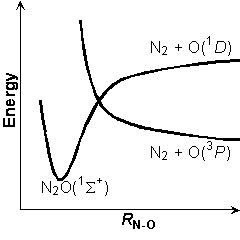
It is supposed that the approach of O(1D) toward the F end of CF3CN is not effective in the quenching process. That is suggested by the fact that the CF4 molecule is quite inert to quenching of O(1D). On the other hand, molecular orbitals for the CN end in the CF3CN molecule are similar to those of N2 molecule by which the electronic quenching process of O(1D) is well known. Actually, the quenching rate constants of CF3CN and N2 are similar.
In the mechanism of quenching process by N2, the reaction proceeds via the collision complex N2O as an intermediate. In the quenching process by CF3CN, the O(1D) atom can approach the N end on the singlet PES leading to CF3CNO(S0). The triplet PES of CF3CNO(T1) correlating to CF3CN + O(3P) intersects this singlet PES. At the seam of the intersection the intersystem crossing, i.e. S0 → T1, should occur. Therefore it is proposed that the CF3CNO intermediate is formed in the quenching reaction process.